Heike Borth1 ・ Anna Teubert2 ・ Ralf Glaubitz2 ・ Sarah Knippenberg2 ・ Nargul Kutur1 ・ Thomas Winkler1 ・ Bernd Eiben1
受理:2020年4月22日/受理:2020年10月20日
©著者(2020年代)
summary
Purpose
Noninvasive prenatal testing (NIPT) is a sensitive and specific method for detecting fetal chromosomal aneuploidies from maternal plasma.The aim of this study was to determine the performance of a new paired-end sequencing-based NIPT assay in 13,607 pregnancies from a single center in Germany.
method
Samples from 13,607 pregnant women who previously underwent NIPT were analyzed for the presence of common fetal trisomies and monosomy X using the VeriSeq NIPT Solution v2 assay and followed up to determine clinical truth.
result
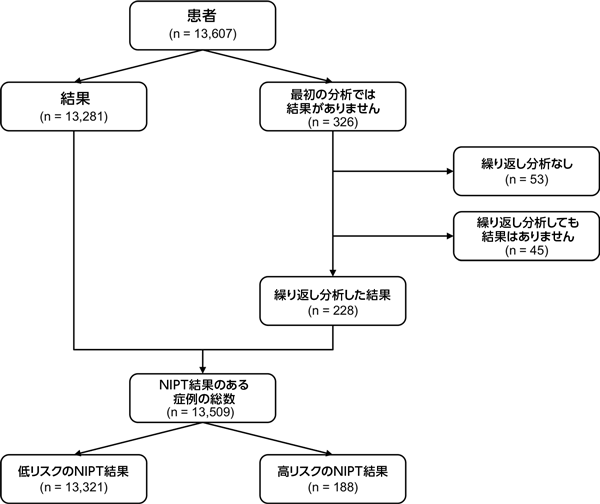
Of the 13,607 cases, 13,509 received an NIPT call, resulting in a low study failure rate of 0.72%. High-risk calls were 188 (1.4%) for trisomy 21 117, trisomy 18 34, trisomy 13 23, trisomy 21 + 13, and monosomy X 13, with high sensitivity and specificity of ≥98.89% reported for all four aneuploidy conditions. Among high-risk cases, clinical follow-up data were available for 77.1% (145/188). For clinical follow-up of high-risk calls, the overall positive predictive value was 84.8% (potential range 65.4–88.3%). NIPT results were provided for samples with fetal fraction ranging up to 2% of the fetal fraction.
in conclusion
The VeriSeq NIPT Solution v2 assay detected fetal chromosomal aneuploidies across a range of fetal fractions with high sensitivity and specificity observed based on known clinical outcomes, high overall PPV, and low failure rates.
keyword
Non-invasive prenatal testing · Fetal chromosomal aneuploidy · Fetal fraction · Positive predictive value · VeriSeq NIPT solution
Introduction
The discovery of fetal cell-free DNA (cfDNA) in maternal circulation in 1997 [1] prompted the development and commercial availability of non-invasive prenatal testing (NIPT) methods to screen for the presence of fetal chromosomal abnormalities. These NIPT assays have been shown to outperform traditional serum screening methods [2], and a recent meta-analysis revealed that NIPT can detect at least 98% of common fetal trisomies in singleton pregnancies with a false positive rate of 0.13% [3]. The use of NIPT in the general pregnant population is supported by many professional societies, including the German Society of Human Genetics [4-7], and recommendations for prenatal screening, including cfDNA screening, were recently published by the German Society of Ultrasound in Medicine (DEGUM), the Austrian Society of Ultrasound in Medicine (OGUM), the Swiss Society of Ultrasound in Medicine (SGUM) and the German Foundation for Fetal Medicine (FMF) [8]. The 2015 Austrian-Swiss-German guidelines for NIPT support the use of NIPT to screen all pregnant women for fetal trisomy 21 but do not recommend screening for sex chromosome aneuploidies or microdeletions.[9] The guidelines note that the performance of cfDNA screening for trisomy 13 and trisomy 18 is lower than that observed for trisomy 21. Germany’s publicly funded insurance system plans to cover NIPT for common fetal trisomies in pregnancies with increased surveillance needs and special risks in the near future.
The range of conditions that NIPT assays can screen for has expanded since their commercial availability in 2011. Initially, NIPT assays screened for the presence of only the common fetal trisomies, namely trisomy 21, trisomy 18, and trisomy 13 [10, 11]. This was then expanded to include optional testing for fetal sex and sex chromosome aneuploidies [12, 13], and subsequently to include screening options for common microdeletions [14, 15], rare autosomal aneuploidies, and partial deletions and duplications [16-18]. Additional fetal anomalies captured after genome-wide testing may impact patient management, as they are often associated with severe pregnancy complications [16, 19]. The ability of commercially available NIPT assays to detect additional fetal anomalies depends on the technology used in the assay. The current major NIPT techniques include massively parallel whole genome next-generation sequencing (NGS) [20-22], single nucleotide polymorphism (SNP)-based targeted sequencing [23, 24], and microarray sequencing [25], of which only a few NGS-based assays have been currently validated to detect rare autosomal aneuploidies and partial deletions and duplications. Massively parallel sequencing techniques can use either single-end or paired-end sequencing; the use of paired-end sequencing is advantageous because it provides information on both DNA fragment size and location. Several studies have demonstrated the use of this type of sequencing for noninvasive screening of fetal chromosomal aneuploidies [26, 27], and the first paper on the clinical use of paired-end sequencing was published in 2017 [28].
The fetal fraction (FF) of pregnancy plasma samples has been shown to play an important role in NIPT [29]. Patient characteristics can affect the fetal fraction level of NIPT samples, with a positive correlation between gestational age and fetal fraction and a negative correlation between maternal weight and fetal fraction shown [24, 30, 31]. Low fetal fraction has been found to be one of the main reasons for NIPT assay failure. Studies have also shown that samples with very low fetal fraction are at higher risk of aneuploidy, especially trisomy 18 and trisomy 13 [23, 32-34]; this is important because some NIPT assays apply a fetal fraction cutoff, which may miss many affected pregnancies.
Our laboratory previously reported on the performance of a SNP-based NIPT assay in the analysis of cfDNA in 3000 cases [35]. The aim of this study was to determine the performance of the VeriSeq™ NIPT Solution v2 assay, a paired-end sequencing-based NGS assay, in testing for fetal chromosomal aneuploidies in 13,607 general pregnancy samples. We also wanted to investigate the role of fetal fraction in our study and determine whether patient characteristics influenced the study results.
Electronic supplementary information
The online version of this article ( Analysis of cell-free DNA in a consecutive series of 13,607 routine cases for the detection of fetal chromosomal aneuploidies in a single center in Germany ) contains supplementary material, which is available to authorized users.
- Bernd Eiben
eiben@eurogen.de
- 1 Amedes Institute of Experimental Medicine and Clinical Genetics Rain/Ruhr, Willy-Brandtplatz 4, Essen, Germany
- Amedes Genetics, Georgstr. 50, 30159 Hannover, Germany
Materials and methods
Subject/specimen details
This retrospective analysis study included 13,607 consecutive patients who underwent NIPT from the general pregnancy population collected over a 17-month period from December 2017 to April 2019. Both singleton and twin pregnancy samples ≥10 weeks gestation were included in the study; exclusion criteria included known missing twins or high-grade multiple pregnancies. In this study, existing sequencing files from the original sample analysis were reanalyzed using the bioinformatics pipeline of a new NIPT assay performed in the laboratory, the VeriSeq NIPT Solution v2. The results of the reanalysis were not communicated to patients or physicians. Amedes adheres to the provisions of the Federal Data Protection Act. Patient consent was obtained from all patients involved in the study in order to use their data for proper quality control and improvement of the NIPT assay. Furthermore, all data were de-identified before inclusion in the study.
Indications for NIPT included advanced maternal age, positive screening test results (ultrasound, serum markers), other medical reasons, or patient anxiety. Other medical reasons included known diseases such as diabetes, epilepsy, cancer, medications such as chemotherapy, history of pregnancy complications including miscarriage or previously affected pregnancy (e.g., monosomy 21, 18, 13, monosomy X), family genetic abnormalities (e.g., trisomy 21), or consanguinity. In the absence of information on the indication, patient anxiety or advanced maternal age (if the patent was 35 years or older) was used as the indication. In the case of multiple indications among the above indications, they were assigned to the indication groups, with the priority being to assign each patient to only one indication group, such as 1. positive screening test, 2. advanced maternal age, 3. other medical reasons, or 4. patient anxiety. The accuracy of the classification of cases into the different indication groups, as well as feedback on all other information of the patient’s medical history and clinical outcome, depended on the accuracy of the information received.
Clinical outcomes of the study cases, i.e., clinical truth, were determined by invasive prenatal diagnostic techniques (cytogenetic analysis after chorionic villus sampling (CVS) and/or amniocentesis), products of conception analysis (cytogenetic analysis of aborted tissue or placenta samples, post-mortem examination such as autopsy or gross evaluation of abortion), and postnatal cytogenetic analysis, as well as ultrasound and neonatal physical examination. A positive NIPT result for fetal aneuploidy was considered confirmed if it was validated by either an invasive prenatal diagnosis or an abnormality observed on ultrasound consistent with a high-risk NIPT call. A low-risk NIPT result was considered confirmed if the attending physician provided feedback for a healthy liveborn infant not clearly compatible with trisomy 21, 18, 13, or monosomy X (e.g., clubfoot, renal pelvic dilation, cardiac defects such as ventricular septal defect, or intrauterine growth restriction due to placental insufficiency).
VeriSeq NIPT Solution v2 Assay
NIPT analysis was performed using the VeriSeq NIPT Solution v2 bioinformatics pipeline (Illumina Inc., San Diego, CA, USA). This analysis uses paired-end sequencing and has two reporting modes: “basic”, which reports on common trisomies and sex chromosomes (if selected), and genome-wide analysis to detect the presence of genome-wide fetal anomalies (including rare autosomal aneuploidies and partial deletions and duplications of 7 Mb or more). As patients initially consented to analysis of their samples for common trisomies and sex chromosomes (if selected), no findings were reported in this study for abnormalities other than those screened for using the basic mode. After analysis of existing sequencing files with the VeriSeq NIPT Assay Software v2, samples were called abnormality detected or no abnormality detected for common fetal trisomies (trisomy 21, trisomy 18, and trisomy 13) and monosomy X (singletons only). Fetal fraction estimates for the samples in question were also provided.
The fetal fraction was estimated using the observed coverage data and fragment size distribution. Coverage-based estimates were obtained using a method similar to that previously described [36]. Size-based FF estimates were based on the fact that fetal cfDNA fragments are, on average, shorter than maternal fragments [27, 36, 37].
After data analysis, a log-likelihood ratio (LLR) score was provided for each sample, which is the probability that a sample is affected given the sample’s estimated FF and observed coverage. The analysis software also used a dynamic threshold metric known as the individualized fetal aneuploidy confidence test (iFACT), which determines whether there is sufficient sequencing coverage for each individual sample given the FF estimate for that sample; samples that did not meet this threshold were reported as QC failures. Other QC metrics, such as coverage uniformity, were also taken into account. The test method also provided T-Statistics values, which helped distinguish low-risk from high-risk samples.
統計
Statistical data analysis was performed using Microsoft Excel 2010. Where applicable, statistical significance of differences was assessed by Student’s t-test, and a p-value <0.05 was considered significant. Binomial 95% confidence intervals (CIs) were calculated for sensitivity and specificity estimates.
In case of inconclusive NIPT results of the first blood sample, a second blood sample was taken for reanalysis. In such cases, the results of the first analysis were excluded from the statistical analysis, and only one result per patient was included in the statistical evaluation. Also, in some patients where the analysis of the first blood specimen gave an NIPT result but showed a low FF of less than 4% (3% FF or 2% FF), another blood sample was taken for reanalysis, which was treated as a goodwill gesture. Again, the results of the first analysis were excluded from the statistical analysis, unless the reanalysis did not give an NIPT result, in the latter case the results of the first analysis were included in the statistics for 2% or 3% FF, excluding the failed reanalysis.
result
A total of 13,607 pregnancy cases were included in the study, including 13,333 singleton and 274 twin pregnancy samples. Of these, 13,281 (97.6%) had NIPT results upon analysis of the first blood sample. Of the 326 samples that did not yield results using the first blood sample, 273 underwent repeat analysis with the second blood sample, and 228 (83.5%) cases obtained results. Overall, a total of 13,509 patients had NIPT results (Fig. 1). The study failure rate was 0.72% (98/13,607). Clinical outcomes were available for 2623 cases. Results were based on invasive diagnostic techniques, ultrasound, or neonatal physical examination (Supplementary Table 1; Online Resource 1).
Patient demographics of the 13,607 patients included in the overall study are shown in Table 1. Nearly half of all patients chose to undergo NIPT initially due to parental anxiety. The mean fetal fraction in the study sample was 9.62%, with a range of 1.20–33.94%. The relationship between fetal fraction and patient BMI is shown in Figure 2. Overall, 86.6% of patients were tested in the first trimester. The relationship between gestational age and other patient characteristics (maternal age, BMI, fetal fraction) is shown in Supplementary Figure 1 (Online Resource 2). As expected, BMI increased with increasing gestational age, as did fetal fraction. A notable increase in fetal fraction was observed only from ≥20 weeks of gestation, whereas only a significant but small increase in fetal fraction was observed in the earlier weeks of gestation. The relationship between the indication for screening and maternal age, BMI, and fetal fraction was also explored (Supplementary Figure 2; Online Resource 2).
Of the 13,509 patients who received NIPT results, 98.6% (13,321) were called low risk and 1.4% (188) were called high risk for the presence of fetal chromosomal aneuploidy (Fig. 1). Clinical outcomes were available for 2623 cases (Supplementary Table 1; Online Resource 1). Of the 13,321 cases reported as low risk for fetal aneuploidy, clinical outcomes were available for 2478 cases (18.6%). Of these, there was one false-negative call that turned out to be a case of trisomy 21. The proportion of fetuses in this false-negative case was 3%.
Of the 188 high-risk calls, 117 were trisomy 21, 34 were trisomy 18, 23 were trisomy 13, one was 21 + 13, and 13 were trisomy X calls. 1.3% of patients in this study received a positive NIPT call for the presence of classical trisomies. Follow-up was available for 77.1% (145/188) of these high-risk cases. As shown in Table 2, based on 123 true-positive cases and 22 false-positive cases, a sensitivity of ≥98.89% and a specificity of ≥99.73% were observed for high-risk cases. Results for one case of 21 + 13 trisomy are not shown, but this case was found to be true positive. The overall PPV for high-risk cases was 84.8% (123/145). PPV results for trisomy 21, trisomy 18, trisomy 13, and trisomy X cases are shown in Table 2. We calculated the theoretical PPV range based on the assumption that all high-risk cases without follow-up were true positive or all true negative. The theoretical PPV range for high-risk cases was 65.4% (123/188) to 88.3% (166/188). Stratification of NIPT results by maternal age, BMI, and FF is shown in Supplementary Fig. 3 (Online Resource 2). Trisomy 18 and trisomy 13 were found to be associated with significantly lower fetal fractions (p < 0.01 and p < 0.001, respectively, compared with low-risk NIPT results). The fetal fractions of all low- and high-risk NIPT cases stratified by patient BMI are shown in Supplementary Fig. 4 (Online Resource 2). LLR scores and T-statistics for true-positive and false-positive high-risk cases are shown in Supplementary Table 2 (Online Resource 1). As can be seen, both the LLR scores and T-statistics were much lower in the false-positive trisomy 21 cases than in the true-positive trisomy 21 cases.
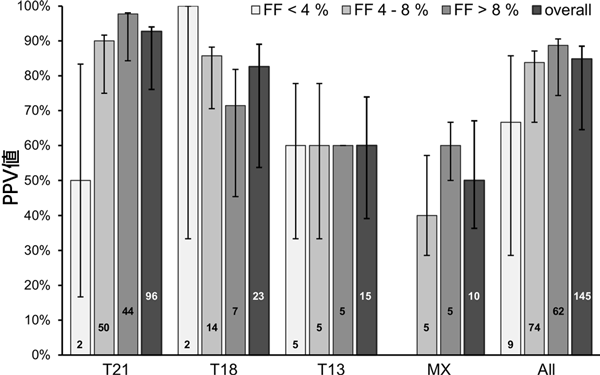
Overall, 2.0% (264/13,509) of samples had a fetal fraction <4%, 43.9% (5933/13,509) of samples had a fetal fraction between 4 and 8%, and 54.1% (7312/13,509) of samples had a fetal fraction >8%. A breakdown of high-risk NIPT results by fetal fraction is shown in Figure 3. As can be seen, the majority of high-risk cases had a fetal fraction between 4 and 8% (49.5%; 93/188). Of the 264 samples with a fetal fraction <4%, 21 were classified as high-risk by NIPT (trisomy 21 6, trisomy 18 6, and trisomy 13 9). Results were available in 9 of these 21 cases, with a PPV of 66.7% (6/9). In low-risk cases, an NPV of 98.1% (52/53) was observed in cases with a fetal fraction of <4%, whereas an NPV of >99.9% was observed in cases with a fetal fraction of 4–8% (1198/1198) and in cases with a fetal fraction of ≥8% (1227/1227).
| Study cohort (n = 13,607) | |
| Mother’s age (years) | |
|---|---|
| Mean ± standard error | 33.68 ± 0.04 |
| Range | 17.45–55.83 |
| gestational age | |
|---|---|
| Mean ± standard error | 12.48 ± 0.02 |
| Range | 10.00–36.57 |
| BMI | |
|---|---|
| Mean ± standard error | 24.87 ± 0.05 |
| Range | 15.05–60.96 |
| Screening indication[n(%)] | |
|---|---|
| Mother’s advanced age | 5755 (42.3) |
| Screening test positive result | 819 (6.0) |
| Other medical tests | 748 (5.5) |
| Patient anxiety | 6285 (46.2) |
Year, week, SEM standard error of the mean, BMI body mass index
Other medical reasons for a positive screening test result, including ultrasound or serum marker screening, include
, for example, ultrasound abnormalities, the patient’s known disease (e.g., diabetes, epilepsy, and carcinoma), medications (e.g., chemotherapy), or high-risk family history such as a history of miscarriage, genetic abnormalities in a previous pregnancy (e.g., trisomy 21, monosomy 18, monosomy 13 X), genetic abnormalities in family members (e.g., trisomy 21), or consanguinity.
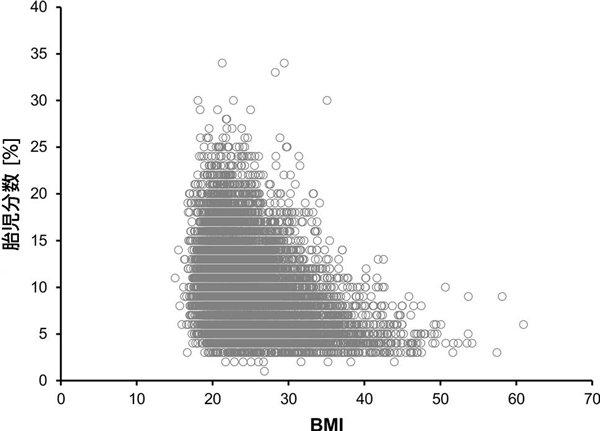
Table 2: Sensitivity, specificity, and positive predictive value for high-risk NIPT cases
| Trisomy 21 | Trisomy 18 | Trisomy 13 | Monosomy X | |
|---|---|---|---|---|
| Number of cases (n) | 117 | 34 | 23 | 13 |
| Follow-up investigation example [n] | 96(82.1) | 23(67.6) | 15(65.2) | 10(76.9) |
| Sensitivity[%(n/N;95%)] | 98.89(89/90; 93.96–99.97) | > 99.99(19/19; 82.35–100) | > 99.99(9/9; 66.37–100) | > 99.99(5/5; 47.82–100) |
| 特異度[%(n/N;95%)] | 99.73(2566/2573; 99.44–99.89) | 99.84(2496/2500; 99.59–99.96) | 99.76(2486/2492; 99.48–99.91) | 99.80(2482/2487; 99.53–99.93) |
| PPV [%(n/N)] | 92.7(89/96) | 82.6(19/23) | 60.0(9/15) | 50.0(5/10) |
| Theoretical low PPV [%(n/N)] | 76.1(89/117) | 55.9(19/34) | 39.1(9/23) | 38.5(5/13) |
| Theoretical top PPV [%(n/N)] | 94.0(110/117) | 88.2(30/34) | 73.9(17/23) | 61.5(8/13) |
Investigation
Here, we show that the VeriSeq NIPT Solution v2 assay was able to screen fetal chromosomal aneuploidies with high sensitivity and specificity observed based on known clinical outcomes. The sensitivity and specificity reported in our study are consistent with those found in a recent meta-analysis by Gil et al. [3] In our study, the overall failure rate was also low, less than 1%. For clinical studies, the positive predictive value is an important indicator that should be reported along with the sensitivity and specificity of the assay, if clinical follow-up is available. Here, clinical truth was available for the majority of high-risk cases. In our study, we observed a high PPV, especially for trisomy 21 and trisomy 18 cases, at a level similar to that shown in previous studies [22, 24, 38]. In the limited follow-up of low-risk cases in our study, we found one false-negative case that was determined to be a trisomy 21 case. We
also examined the influence of patient characteristics on the study results. A positive correlation was observed between gestational age and fetal proportion, which has been noted in several previous studies [24, 30, 31]. As expected, we found a positive correlation between gestational age and BMI. We also noted a significantly lower proportion of fetuses in cases that screened positive for trisomy 18 and trisomy 13. Because the cfDNA analyzed by the NIPT assay originates from the placenta, the previously reported small placental volume during pregnancy may have influenced either trisomy 18 or trisomy 13 [39] and may be responsible for the reduced FF levels observed in studies of these trisomy conditions.
One of the strengths of our study is that it involved a large cohort of patients from the general pregnancy population, providing further evidence of the utility of NIPT in that patient population. We show high test performance of this NIPT assay, with high sensitivity, specificity, and PPV reported for NIPT-positive cases. We also report a low assay failure rate of <1% after retesting a second blood sample for samples with no results on the first blood sample. A low failure rate is an important feature for an NIPT assay, as it avoids the need for patients to decide whether to undergo invasive diagnostic testing in the absence of an NIPT result, which can increase parental anxiety. Professional societies often recommend that failed NIPT samples should be considered high risk [4, 40, 41]. A limitation of our study was our inability to report on additional findings of the genome-wide assay due to the retrospective nature of the study. This meant that we were unable to provide performance data related to screening for rare autosomal aneuploidies and partial deletions and duplications. The ability of this NIPT assay to screen for fetal chromosomal aneuploidies even at very low fetal fractions is important, as low fetal fractions have been shown to be associated with adverse outcomes [42]. However, the small sample size in our study with fetal fractions below 4% did not allow a meaningful analysis of FF performance at these low levels. Another limitation of this study was the limited outcome information for cases with negative NIPT results, which is consistent with many other published NIPT studies [22, 24].
Currently, there is no insurance coverage for NIPT in Germany, so patients must pay out-of-pocket costs for NIPT, which can often be prohibitive. However, the German publicly funded health insurance system plans to cover NIPT for trisomies 21, 13, and 18 for certain pregnancies and special-risk pregnancies with an increased need for surveillance. The introduction of nationwide health insurance for NIPT has been shown to significantly increase the renewal of this type of prenatal screening for women at high risk for fetal aneuploidies [43, 44]. It is important to inform patients that NIPT is not a diagnostic test and that high-risk NIPT calls must be confirmed by invasive diagnostic procedures such as CVS or amniocentesis. Patients found to be at high risk after an abnormal ultrasound should be encouraged to undergo invasive diagnostic procedures to avoid false NIPT results that may occur for reasons such as limited placental mosaicism [45].
In conclusion, the VeriSeq NIPT Solution v2 assay showed a strong performance for the detection of fetal chromosomal aneuploidies in our large population.
Acknowledgements
The authors thank Cosmin Deciu and Sarah Kinnings (Illumina) for their collaboration on this project, and Kristine Jinnett and Kirsten Curnow (Illumina) for their help in preparing the manuscript. Wolf Kupatt and Christoph Keck (amedes) have always supported and advanced the NIPT project in an excellent way. We also thank the many gynecologists who provided valuable information on fetal outcomes.
Author contributions
Data analysis and paper preparation were performed by HB and BE. Assistance with data analysis was provided by NK, AT, and SK. Paper preparation and discussion were assisted by RG and TW.
Funding
Assistance with data analysis and paper preparation was provided by Illumina.
Compliance with ethical standards
Conflicting interests
The authors declare that they have no conflicts of interest.
倫理的承認
Amedes complies with the provisions of the Federal Data Protection Act.
I agree to participate
Patient consent was obtained from all patients involved in the study in order to use their data for proper quality control and improvement of the NIPT assay. Furthermore, all data were anonymized before being included in the study.
Open Access
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source. You provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
References
- Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS (1997) Presence of fetal DNA in maternal plasma and serum. Lancet 350(9076):485-487. https://doi.org/10.1016/s0140-6736(97)02174-0
- Norton ME, Baer RJ, Wapner RJ, Kuppermann M, JellifPawlowski LL, Currier RJ (2016) Cell-free DNA versus sequential screening for the detection of fetal chromosomal abnormalities. Am J Obstet Gynecol 214(6):727–726. https://doi.org/10.1016/j.ajog.2015.12.018
- Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH (2017) Analysis of cell-free DNA in maternal blood in screening for aneuploidy: an updated meta-analysis. Ultrasound Obstetrics and Gynecology 50(3):302-314. https://doi.org/10.1002/uog.17484
- Benn P, Borrell A, Chiu RW, Cuckle H, Dugoff L, Faas B, Gross S, Huang T, Johnson J, Maymon R, Norton M, Odibo A, Schielen P, Spencer K, Wright D, Yaron Y (2015) Position statement of the Chromosome Abnormality Screening Committee. Gestation 35(8):725-734. https://doi.org/10.1002/pd.4608
- Gregg AR, Skotko BG, Benkendorf JL, Monaghan KG, Bajaj K, Best RG, Klugman S, Watson MS (2016) Noninvasive prenatal screening for fetal aneuploidy, revised 2016: American College of Medical Genetics and Genomics position statement. Gene satisfies 18(10):1056-1065. https://doi.org/10.1038/ gim.2016.97
- Dondorp W, de Wert G, Bornchi DW, Bianchi DW, Bergmann C, Borry P, Chitty LS, Fellmann F, Forzano F, Hall A, Howard HC, Lucassen A, Ormond K, Peterlin B, Radojkovic D, Rogowski W, Soller A, Tibben A, Tibben Jaerg L, Tranebjaerg L, van El CG, Cornel MC, European Society of Human G, American Society of Human G (2015) Non-invasive prenatal testing for aneuploidy and beyond: challenges for responsible innovation in prenatal screening. Eur J Hum Genet 23(11):1438–1450. https://doi.org/10.1038/ejhg.2015.57
- Deutscheme Deutscheft Humangenetik analyzes DNA using MUTterlichen Blut. https://www.⦁ gfhev.de/leitlinien. 2013.
- Kozlowski P, Burkhardt T, Gombruch U, Gonser M, Kahler C, Kagan KO, von Kaisenberg C, Klaritsch P, Merz E, Steiner H, Tercanli S, Vetter K, Schramm T (2019) DEGUM, OGUM, SGUM , FMF Germany recommendations, detailed ultrasound examinations, cell-free DNA screening and diagnostic procedures. Ultraschall Med 40(2):176–193. https://doi.org/10.1055/a-0631-8898
- Schmid M, Klaritsch P, Arzsch W, Burkhardt T, Duba HC, Hausler M, Hafner E, Lang U, Pertl B, Speicher M, Steiner H, Tercanli S, Merz E, Heling KS, Eiben B(2015) Cell-free DNA testing for fetal chromosomal abnormalities in clinical practice: Austria-German-Swiss non-invasive prenatal testing (NIPT) recommendations. Ultraschall Med 36(5):507–510. https://doi.org/10.1055/s-0035-1553804
- Nicolaides KH, Syngelaki A, Ashoor G, Birdir C, Touzet G (2012) Non-invasive prenatal testing for fetal trisomy in a routinely screened first trimester population. Am J Obstet Gynecol 207(5):374.e371-376. https://doi.org/10.1016/j.ajog.2012.08.033
- Palomaki GE, Deciu C, Kloza EM, Lambert-Messerlian GM, Haddow JE, Neveux LM, Ehrich M, van den Boom D, Bombard AT, Grody WW, Nelson SF, Canick JA (2012) Maternal plasma DNA sequences. Decision positively identifies trisomy 18 and 13 and Down syndrome: an international collaborative study. Gene satisfies 14(3):296-305. https://doi.org/10.1038/gim.2011.73
- Samango-Sprouse C, Banjevic M, Ryan A, Sigurjonsson S, Zimmermann B, Hill M, Hall MP, Westemeyer M, Saucier J, Demko Z, Rabinowitz M (2013) SNP-based non-invasive prenatal testing , to detect sex chromosome aneuploidy with high accuracy. Gestation 33(7):643-649. https://doi.org/10.1002/pd.4159
- Mazloom AR, Dzakula Z, Oeth P, Wang H, Jensen T, Tynan J, McCullough R, Saldivar JS, Ehrigh M, van den Boom D, Bombard AT, Maeder M, McLennan G, Meschino W, Palomaki GE, Canick JA, Deciu C (2013) Non-invasive pre-detection of sex chromosome aneuploidies by sequencing circulating asexual cell DNA from maternal plasma. Gestation 33(6):591-597. https://doi.org/10.1002/pd.4127
- Wapner RJ, Babiarz JE, Levy B, Stosic M, Zimmermann B, Sigurjonson S, Wayham N, Ryan A, Banjevic M, Lacroute P, Hu J, Hall MP, Demko Z, Siddiqui A, Rabinowitz M, Gross SJ , Hill M, Benn P (2015) Expanding the reach of non-invasive prenatal testing: Detection of fetal microdeletion syndrome. Am J Obstet Gynecol 212(3):332–339. https://doi.org/10.1016/j.ajog.2014.11.041
- Gross SJ, Stosic M, McDonald-McGinn DM, Bassett AS, Norvez A, Dhamankar R, Kobara K, Kirkizla E, Zimmermann B, Wayham rJE, Babiarz JE, Ryan A, Jinnett KN, Denko Z, Benn P ( 2016) Clinical experience with single nucleotide polymorphism-based noninvasive prenatal screening for 22q11.2 deletion syndrome. Ultrasound Obstetrics and Gynecology 47(2):177-183. https
- https://doi.org/10.1002/uog.15754
- Pertile MD, Halks-Miller M, Flowers N, Barbacioru C, Kinnings SL, Vavrek D, Seltzer WK, Bianchi DW (2017) Rare autosomal trisomy revealed by maternal plasma DNA sequencing and fetal Indicates increased risk of placental disease. Sci Translmed https://doi.org/10.1126/scitranslmed.aan1240
- Fiorentino F, Bono S, Pizzuti F, Duca S, Polverari A, Faieta M, Baldi M, Diano L, Spinella F (2017) Clinical utility of genome-wide non-invasive prenatal screening. ):593-601. https://doi.org/10.1002/pd.5053
- Liang D, Lin Y, Qiao F, Li H, Wang Y, Zhang J, Liu A, Ji X, Ma D, Jiang T, Hu P, Xu Z (2018) Cell-free DNA in over 32,000 women Perinatal outcomes after screening: clinical follow-up data from a single tertiary center. Gestation 38(10):755-764. https://doi.org/10.1002/pd.5328
- Scott F, Bonifacio M, Sandow R, Ellis K, Smet ME, McLennan A (2018) Rare autosomal trisomies: Important and not so rare. Gestation 38(10):765-771. https://doi.org/10.1002/pd.5325
- Sehnert AJ, Rhees B, Comstock D, de Feo E, Heilek G, Burke J, Rava RP (2011) Optimal detection of fetal chromosomal abnormalities by massively parallel sequencing of cell-free fetal DNA from maternal blood Clinkem57 (7):1042-1049 https
- https://doi.org/10.1373/clinchem.2011.165910
- Ehrich M, Zwiefhofer T, Tynan JA, Tynan L, Tim R, Lu V, McCullough R, McCarthy E, Nygren AO, Dean J, Tang L, Hu T, Wang H, Angkachatchai V, Oeth P, Cantor CR , Bombard A, van den Boom D (2011) Non-invasive detection of fetal trisomy 21 by sequencing DNA in maternal blood: a study in a clinical setting. Am J Obstet Gynecol 204(3):205–211. https://doi.org/10.1016/j.ajog.2010.12.060
- Taneja PA, Snyder HL, de Feo E, Kruglyak KM, Halks-Miller M, Curnow KJ, Bhatt S (2016) Non-invasive prenatal testing in the general obstetric population: clinical performance and over 85,000 cases. Counseling considerations in cases. Gestation 36(3):237-243. https://doi.org/10.1002/pd.4766
- Pergament E, Cuckle H, Zimmermann B, Banjevic M, Sigurjonson S, Ryan A, Hall MP, Dodd M, Lacroute P, Stosic M, Chopra N, Hunkapiller N, Prosen DE, McAdoo S, Demko Z, Siddiqui A , Hill M, Rabinowitz M (2014) Single nucleotide polymorphism-based prenatal screening in high- and low-risk cohorts. Obstetrics and Gynecology 124(2 Pt 1):210–218. https://doi.org/10.1097/aog.0000000000000363
- Dar P, Curnow KJ, Gross SJ, Hall MP, Stosic M, Demmko Z, Zimmermann B, Hill M, Sigurjonson S, Ryan A, Banjevic M, Kolacki PL, Koch SW, Strom CM, Rabinowitz M, Benn P (2014) Clinical experience and follow-up with non-invasive prenatal aneuploidy testing based on large single nucleotide polymorphisms. Am J Obstet Gynecol 211(5):527.e521–517. https://doi.org/10.1016/j.ajog.2014.08.006
- Juneau K, Bogard PE, Huang S, Mohseni M, Wang ET, Ryvkin P, Kingsley C, Struble CA, Oliphant A, Zahn JM (2014) Microarray-based cell-free DNA analysis offers a non-invasive prenatal test improve. Fetal Diagnosis 36(4):282-286. https://doi.⦁ org/10.1159/000367626
- Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR (2010) Analysis of size distribution of fetal and maternal cell-free DNA by paired end sequencing Clinchem 56(8):1279-1286 https://doi .org/10.1373/clinchem.2010.144188
- Yu SC, Chan YW, Zheng YW, Jiang P, Liao GJ, Sun H, Akolekar R, Leung TY, Go AT, van Vugt JM, Minekawa R, Oudejans CB, Nicolaides KH, Chiu RW, Lo YM (2014). ) Proc Natl Acad Sci USA 111(23):8583–8588. https://doi.org/10.1073/pnas.14061031111
- Cirigliano V, Ordonez E, Rueda L, Syngelaki A, Nicolaides KH (2017) Performance of the Neobona trial Ultrasound Obstetrics and Gynecology 49(4):460-464. https://doi.org/10.1002/uog.17386
- Hui L, Bianchi DW (2020) Fetal fractionation and non-invasive prenatal testing: What clinicians need to know. Pregnancy Diagnosis 40(2):155-163. https://doi.org/10.1002/pd.5620
- Wang E, Batey A, Struble C, Musci T, Song K, Oliphant A (2013) Effects of gestational age and maternal weight on fetal cell-free DNA in maternal plasma Gestation 33(7):662-666. https://doi.org/10.1002/pd.4119
- Scott FP, Menezes M, Palma-Dias R, Nisbet D, Schluter P, da Silva CF, McLennan AC (2017) Factors influencing cell-free DNA fetal fractionation and test accuracy results J Matern Fetal Neonatal Med . https://doi.org/10.1080/14767 058.2017.1330881
- Ryan A, Hunkapiller N, Banjevic M, Vankayalapati N, Fong N, Jinnett KN, Demko Z, Zimmermann B, Sigurjonson S, Gross SJ, Hill M (2016) Single nucleotide polymorphisms for fetal aneuploidy detection Validation of an enhanced version of non-invasive prenatal testing based on Fetal Diagnosis 40(3):219-223. https://doi.org/10.1159/000442931
- Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, Tomlinson MW, Pereira L, Spitz JL, Hollemon D, Cuckle H, Musci TJ, Wapner RJ (2015) Non-invasive testing for trisomy. Cell-free DNA analysis for. N Engl J Med 372(17):1589-1597. https://doi.org/10.1056/NEJMoa1407349
- Fiorentino F, Bono S, Pizzoti F, Mariano M, Polverari A, Duca S, Sessa M, Baldi M, Diano L, Spinella F (2016)
- Determination of the detection limits of non-invasive prenatal testing methods. Pregnancy 36(4):304-311. https://doi.org/10.1002/pd.4780
- Eiben B, Krapp M, Borth H, Kutur N, Kreiselmailer P, Glaubitz R, Deutinger J, Merz E (2015) Single nucleotide polymorphism analysis of cell-free fetal DNA in 3,000 cases from Germany and Austria. Ultrasound Int Open 1(1):E8-E11. https://doi.org/10.1055/s-0035-1555765
- Kim SK, Hannum G, Geis J, Tynan J, Hogg G, Zhao C, Jensen TJ, Mazloom AR, Oeth P, Ehrich M, van den Boom D, Deciu C (2015) Pregnant women using sequence read counting. Determination of fetal DNA fractions from plasma. Gestation 35(8):810-815. https://doi.org/10.1002/pd.4615
- Chan KC, Zhang J, Hui AB, Wong N, Lau TK, Leung TN, Lo KW, Huang DW, Lo YM (2004) Size distribution of maternal and fetal DNA in maternal plasma. Clin Chem 50(1):88-92 https
- https://doi.org/10.1373/clinchem.2003.024893
- Xu L, Huang H, Lin N, Wang Y, He D, Zhang M, Chen M, Chen L, Lin Y (2019) Non-invasive cell-free fetal DNA testing: 31,515 singleton pregnancies in southeastern China A multicenter follow-up study. Ultrasound obstetrics and gynecology. https://doi.⦁ org/10.1002/uog.20416
- Wegrzyn P, Faro C, Falcon O, Peralta CF, Nicolaides KH (2005) Placental volume measured by three-dimensional ultrasound at 11-13 + 6 weeks of pregnancy: association with chromosomal abnormalities. Ultrasound Obstetrics and Gynecology 26(1):28-32. https://doi.org/10.1002/uog.1923
- American College of Obstetricians and Gynecologists, Society of Obstetrics and Gynecology (2016) Medical Bulletin No. 163 Summary: Screening for Fetal Aneuploidy. Obstetrics and Gynecology 127(5):979-981. https://doi.org/10.1097/aog.0000000000001439
- Salomon LJ, Alfirevic Z, Audibert F, Kagan KO, Paladini D, Yeo G, Raine-Fenning N (2017) ISUOG releases consensus on the impact of cfDNA aneuploidy testing on screening policy and prenatal ultrasound practice Updated statement. Ultrasound Obstetrics and Gynecology 49(6):815-816. https://doi.org/10.1002/uog.17483
- Krishna I, Badell M, Loucks TL, Lindsay M, Samuel A (2016) Adverse perinatal outcomes are more frequent in pregnancies with low fetal fraction on non-invasive prenatal testing. Gestation 36(3):210-215. https://doi.org/10.1002/pd.4779
- Vinante V, Keller B, Huhn EA, Huang D, Lapaire O, ManegoldBrauer G (2018) Impact of national health insurance coverage for non-invasive prenatal testing Int J Gynaecol Obstet 141(2): 189–193. https://doi.org/10.1002/ijgo.12422
- Huang T, Dougan S, Walker M, Armour CM, Okun N (2018) Trends in utilization of prenatal testing services for fetal aneuploidy in Ontario: A descriptive study. Open CMAJ 6(4):E436-E444. https://doi.org/10.9778/cmajo.20180046
- Eiben B (2016) Decline of NIPT-eine kritische Betrachtung Frauenaert 57:567
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
 中文
中文