L. Martinez-Jacobo a C. Cordova-Fletes a, b R. Ortiz-Lopez a, b F. Rivas c C. Saucedo-Carrasco d A. Rojas-Martinez a, b
a Department of Biochemistry and Molecular Medicine, Faculty of Medicine; b Molecular Biology Unit, Genomics and Sequencing, Health Sciences Research and Development Center, Autonomous University of Nuevo Leon, Monterrey; c Occidente General Hospital, Guadalajara Secretariat of the Ministry of Health, State of Jalisco; and d Private Office, Department of Pediatrics, San Luis Potosi, Mexico.
keyword
Array CGH Candidate gene Delineation del(7q) Split hand/foot region
summary
In this study, we present a female patient with a de novo congenital deletion of 7q21.3q31.1, as determined by G-banding and CGH-SNP array . The patient presented with features including psychomotor retardation, congenital severe bilateral glaucoma, cleft palate, and cardiac defects. Microarray assays showed the deleted 12.5 Mb region approximately 88 kb downstream of a region associated with finger and toe anomalies; thus, the patient’s final karyotype was 46,XX.arr 7q21.3q31.1(96,742,140-109,246,085)×1dn. This girl represents the fourth patient described so far to present with congenital glaucoma and a deletion encompassing or overlapping the 7q21.3q31.1 region , confirming the presence of a gene locus or loci associated with such clinical features. According to our results, the predisposition to ocular defects secondary to 7q interstitial deletions may be caused by the concomitant deletion of TAC1, HBP1, and a small cluster of cytochrome P450 genes (subfamily 3A) . This conclusion is supported by the functional role and expression site, as TAC1 is also linked to the functional pathway of the MYOC gene, which has a mutation associated with glaucoma. Furthermore, given that this girl clinically evokes several phenotypes associated with diverse deletions within 7q21q32 , our results and observations provide a general overview of the gene content of the overlapping deletion /phenotype at 7q21.3q31.1 and confirm that loci distal to the DLX genes, including potential regulatory elements downstream from the CUX1 gene and DLX5, are independent of finger and toe malformations.
Copyright 2013 S. Karger AG, Basel
Introduction
Congenital 7q deletions are fairly common but heterogeneous. These deletions involve 7q as a whole (DECIPHER database) and are usually classified as proximal (q11→21), intermediate (q21→31/32) and terminal (q32→qter) [Gibson et al., 1982; Young et al., 1984; Chung et al., 2008].
LM-J. and CC-F. contributed equally to this work.Various deletions within the 7q21q32 segment are frequently associated with finger and toe malformations and multiple clinical features, such as intellectual disability/developmental delay, ear/hearing abnormalities, low birth weight, feeding disorders, abnormal crying, microcephaly, micrognathia, cardiac and palatal defects, recurrent infections, abnormal palmar creases, and eye abnormalities [Young et al., 1984; Riviera et al., 1991; Scherer et al., 1994; Montgomery et al., 2000; Bernardini et al., 2008; Chung et al., 2009; This phenotypic consistency together with the apparent overlap of the deleted regions may in fact represent a recognizable deletion syndrome [Fagan et al., 1989]. Here, we report a girl with a 7q21.3q31.1 deletion and congenital severe glaucoma (without finger or toe anomalies) to refine and provide further insight into the mapping of the ocular phenotype associated with interstitial (q21→31/32) deletions .
Patient description
The patient is a 1-year-old girl, the second child born to unrelated parents. Family history was unremarkable. Fetal growth restriction, short femurs, and placental calcification were detected late in pregnancy, and she was delivered by cesarean section at 35 weeks of gestation due to increased fetal risk. Birth weight was 2300 g (<3rd percentile), height was 43 cm (<3rd percentile), and only Apgar score of 8 (out of 10) was noted. Congenital glaucoma was diagnosed and treated with outflow reconstruction procedures (trabeculotomy, trabeculectomy) on two separate occasions. The patient remains intraocularly hypertensive. Head MRI showed subarachnoid edema, altered myelination, and bilateral optic nerve edema. At 7 months of age, her weight was 4300 g (10th–25th percentile) and her height was 57.3 cm (<3rd percentile). She had large eyes, blue sclerae, large anterior and posterior fontanels, prominent brows, low-set ears with excessively folded helices, outwardly elevated palpebral fissures, obvious canthus hypertelorism, broad nasal tip, hypoplastic nasal alar, short columella, bilateral unilateral cleft palmar, and bilateral fifth finger tips (Fig. 1A). She also had a patent foramen ovale, patent ductus arteriosus, and tricuspid regurgitation. At 1 year of age, she was not stable. She was weakly responsive to auditory stimuli, but followed light stimuli. The patient had no developed teeth and a weak cry. Weight, height, and head circumference were 5,500 g (<3rd percentile), 67.5 cm (<3rd percentile), and 42 cm (<3rd percentile), respectively. Ophthalmologic examination revealed bilateral primary congenital glaucoma characterized by exophthalmos, elevated intraocular pressure, blue sclera, and corneal edema with corneal opacity. The uveal structures, including the iris, were apparently normal, although it was unclear whether the uveal vein was implanted on the iris and perfused by the iris vessels. The patient is currently recovering from frequent respiratory infections.
material and method
Chromosome analysis Initial
cytogenetic analysis of the patient and her parents was performed on GTG-banded metaphase chromosomes obtained from 72-h lymphocyte cultures.Molecular studies were performed on further blood samples taken from the patient and her parents with informed consent.
Array CGH
First, genomic DNA from the patient and his parents was obtained from 3 ml of peripheral blood using the Qiagen Gentra® Puregene Blood core Kit. Medium-density microarray analysis was performed using the Agilent SurePrint G3 Hmn CGH+SNP 4x180K microarray kit (containing approximately 120,000 CGH probes and 60,000 SNP probes with a median spacing of 25 kb). Briefly, genomic DNA (approximately 1 mg) from the patient and his parents and gender-matched controls was digested with AluI and RsaI restriction enzymes (Promega, Madison, Wisc, USA) for 2 h at 37°C, and the digested products were purified and hybridized to Cy3-dU-labeled products using the Sure-tag DNA labeling kit (Agilent Technologies), followed by cleaning according to the Agilent protocol. Each slide was scanned on a Nimblegen MS 200 scanner (Roche), and the resulting images were converted with image conversion software and visualized with Feature Extraction software (Agilent Technologies). Results were analyzed using the ADM-2 aberration algorithm with Agilent Cytogenomics software v.2.5, using the default analysis method – CGH+SNP v2.
result
The patient’s G-banded karyotype was 46,XX,del(7)(q22q22)dn; parental karyotypes were normal. The corresponding microarray assay showed a deleted 12.5-Mb region (genomic location 96,742,140-109,246,085) encompassing 500 markers and 229 genes (including miRNAs and hypothetical proteins), with ACN9 more proximal and C7orf66 more distal (GRCh37/hg19); thus, a partial monosomy for 7q21.3q31.1. The deletion overlaps with the original critical region for deletion
Investigation
In this study, we report the fourth patient worldwide with congenital glaucoma associated with a de novo congenital deletion involving or overlapping the q21.3q31.1 region.
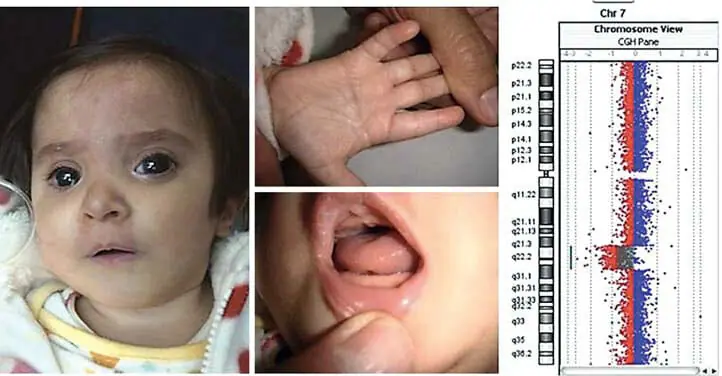

Figure 1
A Maxillofacial, hand and oral characteristics of the patient.
B Loss profile analysis shown as log 2 ratio (plot and line). Visualized with software v.2.5.
C Diagram (non-scale) showing patients with and without ocular abnormalities and carriers of a 7q deletion overlapping 7q21.3q31.1.Most of these chromosomal abnormalities have not been molecularly refined, so we have approximated the breakpoints originally assigned (i.e., we place breakpoints at the proximal or distal end of the bands, rather than at the subbands).Current CasesThe following cases are the ones that overlap primarily with the current deletion among the DECIPHER cases.The vertical blue line indicates the minimum potential area of ocular abnormalities. Showing the minimum area of potential ocular anomalies. The first blue line (left) and the dotted line indicate the area of overlap with our case. The grey line indicates some of the overlapping area. * This case had no reported ocular anomalies or hallux valgus. No hallux valgus was reported. 7q21q31). * * Inexact breakpoint (no hallux valgus). ? = unknown ocular Ocular effect unknown as fetus was not autopsied.
D UCSC browser screenshot All imbalanced cases reported in DECIPHER and ISCA overlap the 7q21.3q31.1 region reported in DECIPHER and ISCA Screenshot with all imbalanced cases reported in DECIPHER and ISCA that overlap the 7q21.3q31.1 region (red = deletion; blue = amplification).) Two deletions in ISCA (IDs nssv578163 and nssv582318) that appear to span the 7q21.3q31.1 region but actually correspond to monosomy 7.
Table 1. Clinical features from cases of deletions reported within 7q21q32, but with clear overlap with the 7q21.3q31.1 region.
| Clinical characteristics | Case cases/references | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 | 12 | 13 | 14 | 15* | 16 | 17 | 今回の事例 | ||||
| General | ||||||||||||||||||||
| – | – | + | – | + | + | + | + | + | + | – | – | + | ||||||||
| ID/DD | + | + | + | + | + | + | + | + | + | + | +a | + | + | +? | +b | + | + | + | ||
| Developmental delay/short stature | ||||||||||||||||||||
| 伸長 | – | – | – | + | + | + | – | + | – | + | – | + | – | + | – | – | – | + | ||
| language delay | – | – | – | – | + | + | td> | + | + | – | – | – | – | – | – | – | –< /td> | – | ? | + |
| 性低血圆 | – | + | – | + | +< /td> | – | – | – | – | + | – | – | + | – | –< /td> | – | – | + | ||
| – | – | + | + | + | – | – | – | – | – | – | – | |||||||||
| 再発性infection | + | + | – | – | + | – | – | – | –? | – | – | – | – | – | – | –? | – | + | ||
| Head/eye socket | ||||||||||||||||||||
| Extensive/pronounced amount around | + | + | – | – | < td>–– | – | – | – | – | – | – | – | – | – | – | – | + | |||
| 小頭症 | + | + | – | – | + | – | – | – | – | – | – | + | – | + | – | – | + | – | ||
| – | – | – | – | – | + | – | – | – | – | – | – | + | ||||||||
| 电影筋のひだ | – | – | – | + | – | – | – | + | – | – | – | – | – | – | – | – | – | NA | ||
| 隆起した眉毛 | – | – | – | – | –< /td> | – | – | – | – | – | – | – | – | – | – | – | – | + | ||
| Hypertelorism/眼角開離 | – | – | – | – | – | – | – | – | – | – | + | – | + | – | + | –? | + | apparently | ||
| Ear/Hearing | ||||||||||||||||||||
| Low set | + | + | – | + | – | – | – | – | – | – | + | – | + | + | – | – | + | + | ||
| 奇形 | + | + | – | + | + | + | + | – | – | – | + | + | + | – | – | – | – | + | ||
| 聴覚障害・聴力障害 | – | – | – | – | – | – | – | – | – | – | – | – | + | – | – | –? | – | apparently | ||
| 鼻 | – | – | – | – | < td>–– | – | – | – | – | + | – | – | – | |||||||
| – | – | + | – | – | + | – | – | – | – | – | – | + | ||||||||
| 眼球の異常 | + | + | G | – | – | + | – | + | + | + | G | + | + | G | ? | –? | – | G | ||
| 口 | ||||||||||||||||||||
| 大 | + | + | – | + | + | – | + | + | – | + | + | – | + | – | – | – | – | – | ||
| 薄い上唖 | – | – | – | + | – | – | – | + | – | ? | ? | – | ? | – | –< /td> | – | – | + | ||
| 長い人中 | + | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | apparently | ||
| 口蓋裂・高口蓋・狭口蓋 | – | – | + | + | – | – | – | – | – | – | – | + | – | + | – | – | – | + | ||
| 小顎症 | + | + | – | – | – | – | – | – | – | + | – | + | + | – | – | – | – | + | ||
| + | + | + | – | + | + | – | + | – | –< /td> | – | – | mildc | ||||||||
| First | ||||||||||||||||||||
| + | – | – | – | < td>–+ | – | – | – | – | –d | – | – | |||||||||
| limbs and hands | ||||||||||||||||||||
| 第5指の無指症 | – | + | – | – | – | – | mild | + | – | – | – | – | – | – | – | – | + | + | ||
| 分裂手指/分裂足 | – | – | – | – | – | – | – | – | – | – | – | + | + | + | – | – | – | – | ||
| Abnormal palmar wrinkles | – | + | – | – | + | – | – | + | – | + | – | – | – | + | – | – | + | |||
| Cardiovascular | ||||||||||||||||||||
| ASD/VSD | – | + | + | – | – | – | – | – | – | – | + | – | + | + | – | – | – | – | ||
| PDA | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + | ||
| 肺動脈狭窄 | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | + | – | – | ||
| – | – | – | – | + | + | – | – | – | + | – | – | +e | ||||||||
| Digestive tube | ||||||||||||||||||||
| ヘルニア | – | – | – | – | – | – | – | + | – | – | – | + | + | – | – | – | – | – | ||
| Urinary system | ||||||||||||||||||||
| 性器異常 | – | – | + | – | – | + | – | + | – | – | – | + | – | + | – | + | – | – | ||
1 Ayraud et al. [1976]; 2 Higginson et al. [1976]; 3 Dennis et al. [1977]; 4 Hull et al. [1979]; 5 Klep-de Pater et al. [1979]; 6 Serup [1980 ]; 7 Abuelo and Padre-Mendoza [1982]; 8 Young et al. [1984]; 9 Chitayat et al. [1988]; 10 Fagan et al. [1989]; 11 Franceschini et al. [1978]; 12 Tajara et al. [1989]; 13 Morey and Higgins [1990]; 14 Montgomery et al. [2000 ]; 15 Cheong et al. [2008]; 16 ID 253694; 17 ID 255298.
ID/DD = Intellectual disability/developmental delay. NA = Not available. G = Glaucoma. ? = Unknown/Incorrect.
Fetus delivered at 22 weeks, no further evaluation is possible. aWe suggest a The one proposed by the authors (early death). c The patient had poor initial attachment. d The patient presented with preaxial polydactyly of the hands.
After this review, a deletion very similar to our case was reported in a 24-week-old fetus, presenting with cleft lip and palate, hyperpigmentation, wide nasal bridge, micrognathia, low-set ears, micropenis, and cryptorchidism [Chen et al. 2013]. In this table, features in more than 30% of cases include ID/DD (~18/18), eye anomalies (12/18), low birth weight (10/18), ear anomalies (10/18), cardiac anomalies (9/18), macromouth (9/18), feeding problems (9/18), low-set ears (8/18), growth retardation/short stature (8/18), hypotonia (6/18), microcephaly (6/18), and micrognathia. The feature of a “weak cry” or “abnormal cry” is not included in this table, but was present in approximately 50 patients, including those in this table.
(Figure 1C, Table 1). Our results suggest that co-deletion of at least three eye-related loci (TAC1, CYP3A43 and HBP1) mapped in this region could explain glaucoma and other ocular abnormalities.
Glaucoma, a clinically and genetically heterogeneous condition, is characterized by loss of retinal ganglion cells and atrophy of the optic nerve [Izotti et al. 2011; Mookherjee et al., 2012]. The main clinical criterion of elevated intraocular pressure is usually due to resistance of the trabecular meshwork to aqueous humor [Kennedy et al. 2012]. The trabecular meshwork is located around the base of the cornea and plays a key role in regulating aqueous humor outflow [Izotti et al. 2011]. Trabecular meshwork alterations are frequently found in patients with congenital glaucoma. Congenital glaucoma, cloudy cornea, primary open-angle glaucoma, and juvenile open-angle glaucoma are some of the subtypes of glaucoma, which are associated with the expression of GLC1A or MYOC (1q24.3), CYP1B1 (2p22.2), and CAV1/CAV2 (7q31.2) [Stoilov et al., 1997;Alward et al., 1998;Thorleifsson et al., 2010;Kennedy et al., 2012;
CYP1B1 gene is linked to primary congenital
glaucoma (OMIM 231300) with cytochrome P450-dependent metabolites that regulate corneal transparency and aqueous humor secretion. Furthermore, CYP1B1 was downregulated and CYP26B1 was upregulated in human trabecular meshwork cells with mutant MYOC gene [Kennedy et al., 2012]. These facts suggest that other genes of the cytochrome P450s family may be involved as well. In fact, a small cluster of cytochrome P450 genes at 7q22.1 (CYP3A4, CYP3A5, CYP3A7, CYP3A43) is located in the cytochrome P450 family. Indeed, in our patients, most of the deletions in 7q
associated with glaucoma or other ocular abnormalities such as corneal opacity, megalocornea, or abnormal pupils involved q22 and probably CYP genes [Young et al., 1984; Montgomery et al., 2000]. Of note, CYP3A43 was found to be differentially expressed in human corneal epithelial tissue [Turner et al., 2007]. In general, members of subfamily 3A were found to be expressed in human iris, ciliary body, and cornea [Chang et al., 2008; Vorotinen et al., 2011]. Another candidate gene for glaucoma appears to be TAC1, which was also deleted in our patient and has recently been suggested as a possible physiological biomarker of glaucomatous damage. The TAC1 gene was linked to the functional pathway of the MYOC gene. The MYOC gene is a glycoprotein induced by stress conditions in the trabecular meshwork. Indeed, TAC1 expression was also strongly altered in MYOC mutant cells [Kennedy et al. 2012]. The TAC1 gene (7q21.3) encodes the precursor of the hormone sole (UCSC Genome Browser) that acts as a neurotransmitter, and has been identified as a mechanosensitive gene in the human trabecular meshwork [Kennedy et al. 2012]. Another gene, also deleted in this patient, namely HBP1, is a transcriptional repressor involved in the WNT pathway and expressed in the retina, cornea and ciliary body (EMBL-EBI and GeneCards databases). The WNT pathway is related to intraocular pressure regulation [Kennedy et al. 2012]. At the same time, the HBP1 (high mobility group box transcription factor) gene is similar to HMGB1 (high mobility group box 1 protein), which is known to be an endogenous molecule for signaling retinal injury and inflammatory stress [Lee et al., 2012].
Although the phenotype of 7q21q32 deletion syndrome is diverse, several recurrent clinical features have been observed (Table 1). According to the information available in Table 1, at least 14 features were present in patients with a frequency of 30% or more (Table 1). For example, the child in this case showed at least 11 of these features, including intellectual disability, developmental delay, growth retardation, and craniofacial, cardiac, and ocular defects. Furthermore, breakpoint analysis revealed that the proximal breakpoint of this deletion is located only 88 kb downstream of the DLX5 gene. Haploinsufficiency of DLX5 and DLX6 genes has been implicated as a cause of hallux valgus [Scherer et al., 1994; van Silfhout et al., 2009], thus indirectly confirming that the CUX1 gene (as suggested by Bernardini et al. [2008]) and potential regulatory elements (as suggested by Tzschach et al. [2007]) downstream of DLX5 and ACN9 (between RP11-800O14 and D7S618) are excluded in determining these limb defects.
These results confirm that 7q21.3q31.1 is a region rich in genes important for brain, heart, growth, and eye physiology and development (Table 2), outline the genetic content of deletions and phenotypes overlapping with 7q21.3q31.1, and further confirm that loci distal to DLX genes are unrelated to hallux valgus.
Table 2. 7q21.3q31.1 deleted genes may be associated with some frequent clinical features seen in other similar deletion cases.
| Related | Gene/Protein | References |
|---|---|---|
| 脳/ID/DD | ACHE, ATXNL7, BHLHA15, COG5, GPC2, MLL5*, NPTX2, NRCAM, PNPLA8, RELN, SRPK2, SYPL1, TAC1. THAP5, TMEM130, VGF, NYAP1a | UCSC Genome Browser, Al-Hassnan et al. [2011] Rymen et al. [2012], deciphering ID263273. Vincent et al. [2008], Uliana et al. [2010] |
| Craniofacial changes | MLL5*, PLOD3**, RELN | Al-Hassnan et al. [2011] , Salo et al. [2008] |
| Deafness | GJC3, LHFPL3, MLL5*, PLOD3**, SLC26A4, SLC26A5 | UCSC genome browser, ID263273, Salo et al. [2008] Albert et al. [2006] |
| Eye abnormalities | CLDN15, CYP3A4, CYP3A43, CYP3A5, CYP3A7, HBP1, NRCAM, TAC1, TMEM1 30 | EMBL-EBI, Vorotinen et al. [2011], Lee et al. [2012], Demyanenko et al. [2011], Kennedy et al. [2012] |
| Heart/cardiac defects | MOSPD3, NPTX2, PNPLA8, SRPK2, THAP5 | UCSC Genome Browser, Paul et al. [2004] |
| Gastrointestinal changes/developmental delay/short stature | CLDN15, MUC3A, MUCB, MUC17, MOGAT3 | UCSC Genome Browser, Wada et al. [2013] |
These associations were performed according to gene function and expression location, gene/phenotypic overlap with other cases, or previous associations. Note that some genes may be associated with multiple clinical features. Indeed, several monogenic lesions appeared to be responsible for various clinical features. MLL5* or PLOD3**.
a Deleted in ISCA microdeletion example (ID nssv578204).
These associations were performed according to gene function and expression location, gene/phenotype overlap with other cases, or previous associations. Note that some genes may be associated with multiple clinical features. Indeed, some monogenic lesions appeared to be involved in various clinical features (examples). MLL5 * or PLOD3 ** .
a Deleted in ISCA microdeletion example (ID nssv578204).
Acknowledgements
We thank the patient’s parents for their continued collaboration and Dr. Jose A. Paczka (Ophthalmologist) for his report. This work was partially supported by PROMEP (No. 103.5/11/4330, PAICYT (SA609-10) to C. Cordova-Fletes, PAICYT (No.) SA324-10, FOMIX (Convocatoria M0014-2007-2010, Reg) 068251) CIDICS L. Martinez-Jacobo is supported by a CONACYT scholarship.
References
Abuelo DN, Padre-Mendoza T: Cat-like crying and mental retardation due to interstitial deletion of 7q (7q22 linked to 7q32). J Med Genet 19: 473-476 (1982)
Albert S, Blons H, Jonard L, Feldmann D, Chauvin P, et al: The SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with vestibular aqueduct hypertrophy in Caucasian populations. Eur J Human Genet 14:773-779 (2006)
Al-Hassnan ZN, Al-Bakheet A, Abu-Dheim N, Al-Younes B, Colak D, Kaya N: A novel interstitial microdeletion at 7q22.1 to 7q22.3 detected by array comparative genomic hybridization. Am J Med Genet 155:3128-3131 (2011)
Alward W, Fingert J, Coote M, Johnson A, Lerner S, et al: Mutations in the chromosome 1 gene (GLC1A) are associated with open-angle glaucoma. N Engl J Med 338:1022-1027 (1998)
Ayraud N, Rovinski J, Lambert JC, Galiana: Interstitial deletion of the long arm of chromosome 7. Ann Genet 19:265-268 (1976)
Bernardini L, Palka C, Ceccarini C, Capalbo A, Bottilo I, et al: Complex rearrangements of chromosome 7q21.13-q22.1 confirm the direct deafness locus and suggest new candidate genes. Am J Med Genet A 146A:238-244 (2008)
Chen CP, Chang SJ, Chern SR, Wu PS, Chen YT, et al: Prenatal diagnosis of de novo interstitial deletion of 7q (7q22.1→q31.1) and its related pathology. Gene 521:311-315(2013)
Cheong MLJ, Tsai MS, Cortes R, Harrison MR: Interstitial deletion of chromosome 7q detected by first-trimester Down syndrome screening. Fetal Diagnosis 24:340-344(2008)
Chitayat D, McGillivray BC, Wood S, Kalousek DK, Langlois S, Applegarth DA: Interstitial 7q deletion [46,XX,del(7)(pter→q21.1::q22→qter)] and location of the beta-glucuronidase and cystic fibrosis genes. Am J Med Genet 31:655-661 (1988)
Demyanenko G, Riday T, Tran TS, Dalal J, Darnell EP, et al: NrCAM deletion topographically misdirects thalamocortical axons to the visual cortex and disrupts vision. J Neurosci 31:1545-1558(2011)
Dennis NR, Neu RL, Bannerman RM: Partial monosomy 7q in an infant with multiple anomalies. Am J Hum Genet 29:37A (1977)
Fagan K, Gill A, Henry R, Wilkinson I, Carey B: Summary of interstitial deletions of 7q and exclusion mapping of the beta-glucuronidase gene. J Med Genet 26:619-625 (1989)
Franceschini P, Silengo MC, Davi GF, Santoro MA, Prandi G, Fabris C: Interstitial deletion of the long arm of chromosome 7 46,XX,del(7) (pter q2200:q32 Hum Genet 44:345-348 (1978)
Gibson J, Ellis PM, Forsyth JS: Interstitial deletion of chromosome 7: case report and literature review. Clin Genet 22:256-265 (1982)
Higginson G, Weaver DD, Magenis RE, Prescott GH, Haag C, Hepburn DJ: Interstitial deletion of the long arm of chromosome 7 (7q-) in an infant with multiple anomalies. Clin Genet 10:307-312 (1976)
Hull DR, Kessler KK, Juberg RC: Interstitial deletion of 7q causes growth failure and specific crying: comparison with previously reported deletions of 7q1 and 7q2. Am J Hum Genet 31:97A (1979)
Izzotti A, Longobardi M, Cartiglia C, Sacca SC: Mitochondrial damage in the trabecular meshwork occurs exclusively in primary open-angle glaucoma and pseudoexfoliative glaucoma. PLoS 1 6e14567 (2011)
Kennedy KD, Anithachristy SA, Buie LK, Borras T: Cystatin A: a possible common association with mutant myocilin-induced glaucoma. PloS 1 7:e36301 (2012)
Klep-de Pater JM, Bijlsma JB, Bleeker-Wagemakers EM, de France HF, de Vries-Ekkers CMAM: Two cases with different deletions of the long arm of chromosome 7. J Med Genet 16:151-154 (1979)
Lee J, Hsiao C, Yang I, Chou MH, Wu CL, et al: High-mobility group box 1 protein inhibits advanced glycation endproduct-induced vascular endothelial growth factor A in the rat retinal ganglion cell line RGC-5. Mol Vision 18:838-850(2012)
Montgomery TL, Wyllie J, Oley C: Chylodactyly and glaucoma associated with interstitial deletions of 7q21.2-q31.2. Clin Dysmorphol 9:235-239 (2000)
Mookherjee S, Acharya M, Banerjee D, Bhattacharjeee A, Ray K: Molecular basis of involvement of CYP1B1 in MYOC upregulation and its potential significance in the pathogenesis of glaucoma. PLoS 7:e45077 (2012).
Morey MA, Higgins RR: Ectro-amelia syndrome with interstitial deletion of 7q. Am J Med Genet 35:95-99 (1990)
Pall GS, Wallis J, Axton R, Brownstein DG, Gautier P, et al: A novel transmembrane MSP-containing protein with a role in right ventricular development. Genome 84:1051-1059(2004)
Rivera H, Sanchez-Corona J, Burgos-Fuentes VR, Melendez-Ruiz MJ: 7q22 deletions and deletion index. Genet Couns 2:27-31 (1991)
Rymen D, Keldermans L, Race V, Rregal L, Deconinck N, et al: COG5-CDG: expanding the clinical spectrum. Orphan J Reare Dis 7:94 (2012)
Salo AM, Cox H, Farndon P, Moss C, Grindulis H, et al: Connective tissue disorders caused by mutations in the lysyl hydroxylase 3 gene. Am J Hum Genet 83:495-503 (2008)
Scherer S, Poorkaj P, Allen T, Kim J, Geshuri D, et al: Fine mapping of the autosomal dominant splint hand/split foot locus on chromosome 7, band q21.3- 1. Am J Hum Genet 55:12-20 (1994)
Serup L: Interstitial deletion of the long arm of chromosome 7. Hum Genet 54:19-23 (1980)
Stoilov I, Akarsu N, Sarfarazi M: Identification of three distinct truncating mutations in cytochrome P4501B1 (CYP1B1) as the major cause of primary congenital glaucoma (staphylococcal) in a family linked to the GLC3A locus on chromosome 2p21. Hum Mol Gen 6:641–647 (1997)
Tajara EH, Varella-Garcia M, Gusson AC: Interstitial deletion of the long arm of chromosome 7 and orthodactyly. Am J Med Genet 32:192-194(1989)
Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, et al: Common variants close to CAV1 and CAV2 are involved in primary open-angle glaucoma Nat Genet 42:906-909 (2010)
Turner H, Budak M, Akinci M, Wolosin JM: Comparative analysis of human conjunctival and corneal epithelial gene expression using oligonucleotide microarrays. Invest Ophthalmol Vis Sci 48:2050-2061 (2007).
Tzschach A, Menzel C, Erdogan F, Schubert M, Hoeltzenbein M, et al: Characterization of a 16 Mb interstitial chromosome 7q21 deletion by tiling path array CGH. Am J Med Genet A 143:333-337 (2007)
Uliana V, Grosso S, Cioni M, Ariani F, Papa FT, et al: A 3.2 Mb microdeletion in bands q22.2-q22.3 of chromosome 7 is associated with overgrowth and retardation. Eur J Med Genet 53:168-170 (2010)
van Silfhout AT, van den Akker PC, Dijkhuizenet T, Verheij J, Olderode-Berends M, et al: Split hand-foot malformations due to chromosome 7q abnormalities (SHFM1): functional hemisphericity as a causative mechanism. Eur J Hum Genet 17:1432-1438 (2009)
Vincent JB, Choufani S, Horike S, Stachowiak B, Li M, et al.: A translocation t(6;7)(p11-p12;q22) associated with autism and mental retardation: localization and identification of candidate genes at the breakpoint. Psychiatry Genet 18:101-109 (2008)
Volotinen M, Hakcola J, Pelkonen O, Vapaatalo H, Mmaenpa AJ: Metabolism of ophthalmic timolol: new aspects of an old drug. Basic Clin Pharmacol Tools 108:297-303 (2011)
Wada M, Tamura A, Takahashi N, Tsukita S: Loss of claudins 2 and 15 from mice causes defects in intestinal paracellular Na(+) flux and nutrient transport, leading to malnutrition-related death. Gastroenterology: S0016-5085(2012)
Young RS, Weaver DD, Kukolich MK, Heerema NA, Palmer CG, et al: Terminal and interstitial deletions of the long arm of chromosome 7: a comprehensive review of five new cases. Am J Med Genet 17:437-450 (1984).
Zhang T, Xiang C, Gale D, Carreiro S, Wu E, Zhang EY: Drug transporters and cytochrome P450 mRNA expression at the human ocular barrier: impact on intraocular pharmacokinetics. Drug Metab Dispos 36:1300-1307(2008).
KARGER
e-Mail karger@karger.com www.karger.com/msyCopyright 2013 S. Karger AG, Basel 1661-8769/13/0046-0285 $38.00/0
Augusto Rojas Martinez, MD/DSc
Union of Molecular Biology, Genomica, Center for Scientific Research, Al Saud Civil Society, Collaborative Research Institute
University of Nuevo Leon, Monterrey 64460 (Mexico) Email: arojustomtu@gmail.com
 中文
中文